Here we outline the process for creating new participant records in LabKey.
Note For managing participants in preclinical studies, please see the sub-page, Participants in Preclinical Studies.
Note Please remember that LabKey does not work with Internet Explorer. Please have Javascript enabled to access all features of Managing Participants.
The Participants tab is organized into two main parts:
Individual participant entry is done through the Enter Participant form:
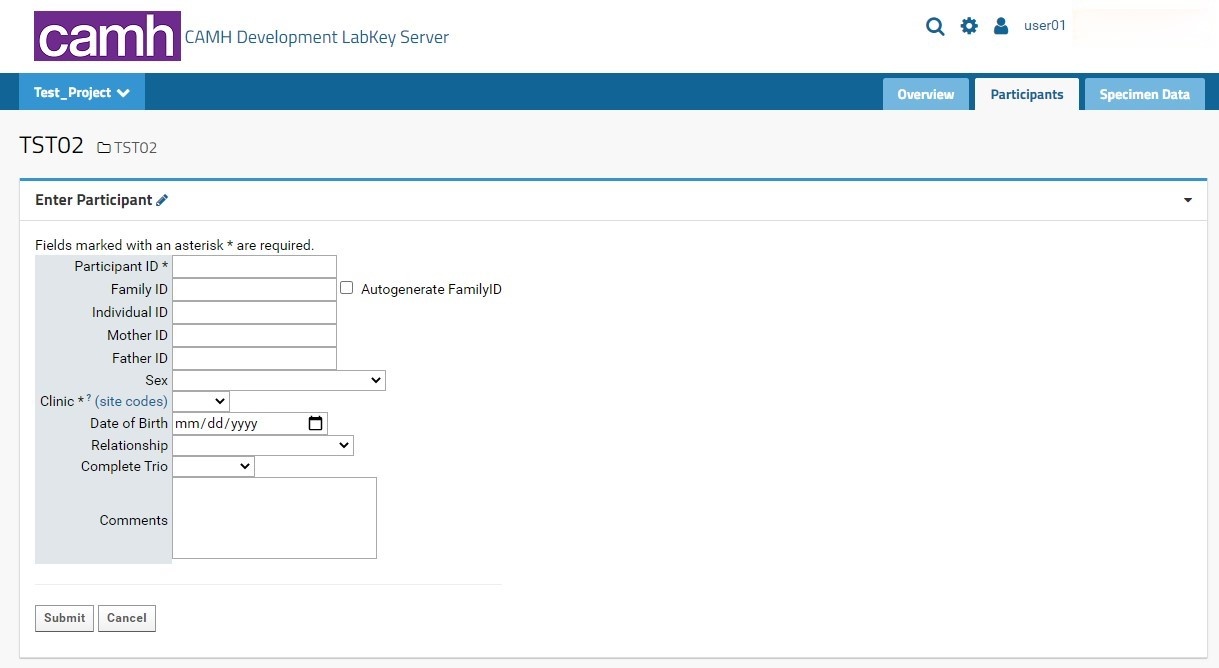
The following table indicates each possible entry field and their expected data types:
Field | Data Type | Comments |
|---|---|---|
Participant ID | Alphanumeric (String) | Required field. Recommended standardized NIP format: PROJECT|STUDYCODE_SITECODE_PARTICIPANTCODE Ex: TST01_CMH_00001000 |
Family ID | Integer | Option to autogenerate a family ID by selecting the checkbox next to the Family ID entry field. This field is mandatory for genetic (DNA/RNA) studies where the study team will be recruiting family members. |
Individual ID | Integer | This field is mandatory for genetic (DNA/RNA) studies where the study team will be recruiting family members. |
Mother ID | Integer | This field is mandatory for genetic (DNA/RNA) studies where the study team will be recruiting family members. |
Father ID | Integer | This field is mandatory for genetic (DNA/RNA) studies where the study team will be recruiting family members. |
Sex | Dropdown menu | Recommended field |
Clinic | Dropdown menu | Required field |
Date of Birth | DateTime | Can be entered manually, or by clicking the calendar icon and selecting the appropriate date. Expected format: MM/DD/YYYY |
Relationship | Dropdown menu | This field is mandatory for genetic (DNA/RNA) studies where the study team will be recruiting family members. |
Complete Trio | Dropdown menu | This field is mandatory for genetic (DNA/RNA) studies where the study team will be recruiting family members. |
Comments | Multi-line textbox | Maximum space of 400 characters |
There are two buttons at the bottom of the form:
Participants can be entered individually or in bulk. The bulk option should only be used by external collaborators who have existing biosample-related data collected and stored elsewhere that are being shared with CAMH scientists for current analysis.
The process for entering single participants is as follows:
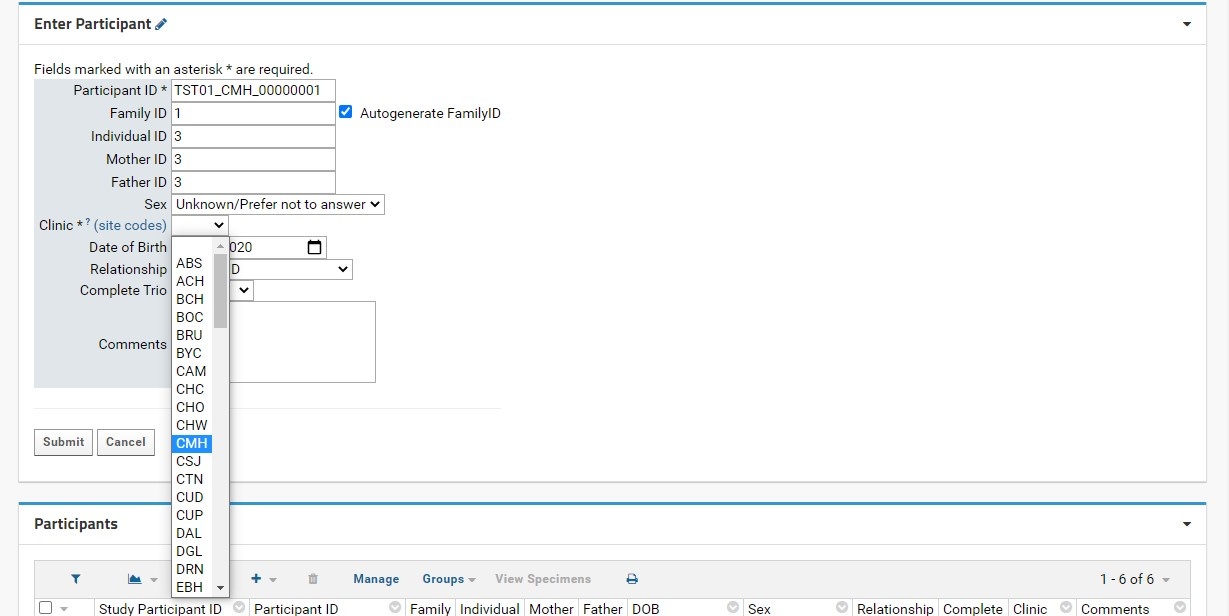
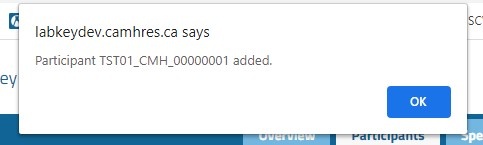
Note Entering participants in bulk has been discontinued. If Participants need to be added in bulk, please contact the LabKey Administrator or Biobank staff.
Note Editing participants by users has been discontinued. If edits need to be made, please contact the LabKey Administrator or Biobank with the following information:
The Administrator or Biobank staff will then add a comment explaining the change request.
Note Deleting participants by users has been discontinued. If deletions need to be made, please contact the LabKey Administrator or Biobank staff.
In certain situations, participants consent to more than one study. In this case, the study team would enter a participant record for each study, noting the Participant ID of the other study to which the participant has consented in the Comments section. For example, the study team would create participant records in both studies ABC and XYZ using the participant’s study-specific participant ID. The study team must also note in the Comments section of the participant record “Same as ABC01_CMH_00000001” in the XYZ record, and “Same as XYZ_CMH_00000001” in the ABC record.
Note that this procedure is similar for specimen entry as well. Please see "Samples Across Multiple Studies" for more details.