Here we outline the process for creating and editing participant records in LabKey for pre-clinical (non-human) studies. Note Please remember that LabKey is not compatible with Internet Explorer. Please have JavaScript enabled to use all features of Managing Participants.
The Participants tab is organized into two main parts:
Individual participant entry is done through the Enter Participant form:
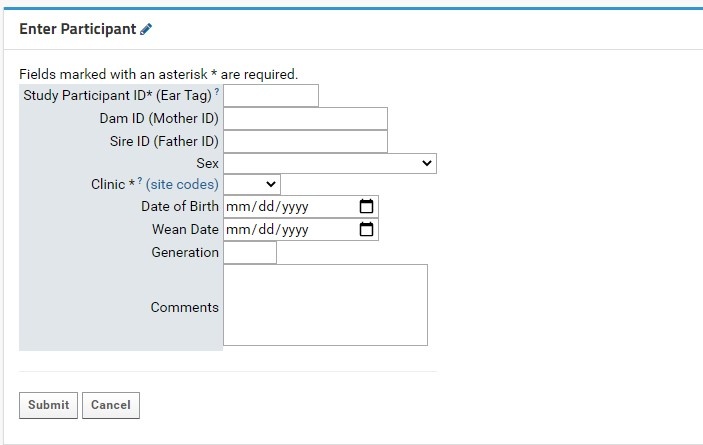
The following table indicates each possible entry field and their expected data types:
| Field | Data Type | Comments |
|---|---|---|
Study Participant ID (Ear tag) | Numeric (Integer) | Required field. Numeric ear tags will be used to generate NIP Participant IDs that may be used in conjunction with other platform software, should it be required. Each tag must be unique to the project, or else LabKey will not create the new Participant record. |
| Dam ID (Mother ID) | Numeric (Integer) | The ear tag of the mother (dam) |
| Sire ID (Father ID) | Numeric (Integer) | The ear tag of the father (sire) |
| Sex | Dropdown Menu | Recommended field |
| Clinic | Dropdown Menu | Required field |
| Date of Birth | Date | Can be entered manually, or by clicking the calendar icon and selecting the appropriate date. Expected format: MM/DD/YYYY |
| Wean Date | Date | Can be entered manually, or by clicking the calendar icon and selecting the appropriate date. Expected format: MM/DD/YYYY |
| Generation | Numeric (Integer) | Value must be between 1 and 30 |
| Comments | Multi-line text | Maximum space of 400 characters |
There are two buttons at the bottom of the form:
Participants can be entered individually or in bulk. Note: the bulk option should only be used when there are over 10 participants to be entered.
The process for entering single participants is as follows:

For the bulk upload of Participants, please fill out the form from the Biobank, and returned the completed document to the Biobank Manager for upload into LabKey.
In the Participants grid view, each Participant can be edited by selecting the pencil icon to the left of the Study Participant ID column. The user will then be taken to the Update Dataset Entry page with the corresponding Participant ID in the top row.

Each field can be edited by typing into empty text boxes or by replacing existing text. Note that each non-dropdown menu option has a superscript question mark (?) next to the field label. This indicates the expected and required data type that must be entered to update the desired participant information.

Note that the Participant ID and Study Participant ID are uneditable, as changing these values may affect linkages with Specimen and Instrument data. If the Participant is entered mistakenly, or if there are errors while entering the Participant ID, it is recommended to delete the participant record (assuming it is not already connected to any specimen records). If updating the Participant or SP-ID is necessary, please contact the LabKey Adminstrator.
In the Participants grid view, each participant can be deleted by selecting the checkbox in the left-most column, and navigating to the top of the grid to click on the DELETE button. An alert box will appear in the browser asking for confirmation to delete the selected rows (participants).

Note that deleting a participant record does not automatically remove the specimens and vials added for that participant; all specimens and vials records associated with the participant record being deleted also need to be deleted manually via the SpecimenDetail webpart.