...
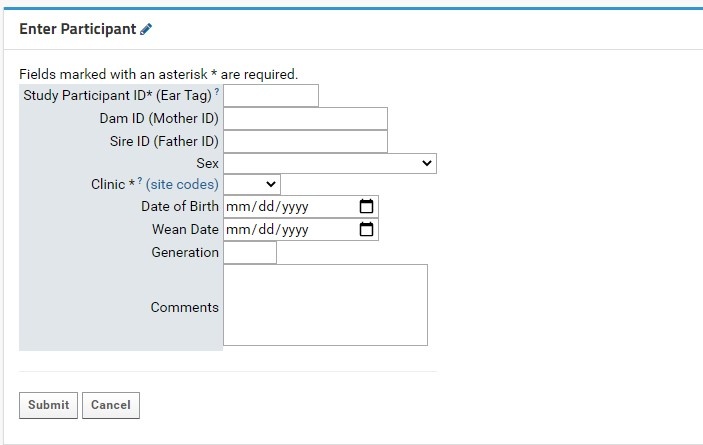
Individual participant entry is done through the Enter Participant form:
- Study Participant ID (Ear tag): this field is required. This field allows for numeric characters only, with a limit of 8 digits in length. This value will be used to generate a LabKey Participant ID, which is standardized across the NIP. A full guide for Participant IDs (LabKey Participant IDs) can be found here.
- Both the Participant and Study Participant IDs will be stored in LabKey for cross-referencing purposes.
- Clinic: this field is required. Users will select the proper KCNI site code where the participants collected from a dropdown list. An external link to shows the full name and location of the three-letter site code for reference.
- Sex: this field is recommended as it can be used for quality control purposes in genetic studies. Sex designations specified by the CAMH patient intake form from a dropdown list. For participants where sex was not entered or is irrelevant for the study’s purpose, it is recommended to mark the field as Unknown/Prefer not to answer.
...
Note that the Participant ID and Study Participant ID are uneditable, as changing these values may affect linkages with Specimen and Instrument data. If the Participant is entered mistakenly, or if there are errors while entering the Participant ID, it is recommended to delete the participant record (assuming it is not already connected to any specimen records). If updating the Participant or SP-ID is necessary, please contact the LabKey Adminstrator.
Deleting Participants
In the Participants grid view, each participant can be deleted by selecting the checkbox in the left-most column, and navigating to the top of the grid to click on the DELETE button. An alert box will appear in the browser asking for confirmation to delete the selected rows (participants).
Note that deleting a participant record does not automatically remove the specimens and vials added for that participant; all specimens and vials records associated with the participant record being deleted also need to be deleted manually via the SpecimenDetail webpart.
...