...
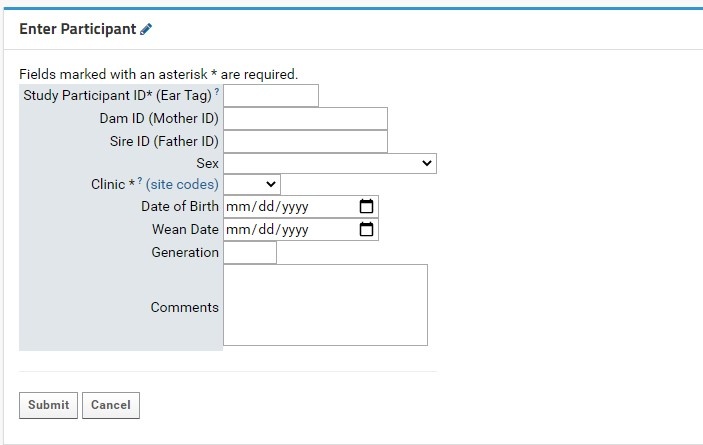
Individual participant entry is done through the Enter Participant form:
- Study Participant ID (Ear tag): this field is required. This field allows for numeric characters only, with a limit of 8 digits in length. This value will be used to generate a LabKey Participant ID, which is standardized across the NIP. A full guide for Participant IDs (LabKey Participant IDs) can be found here.
- Both the Participant and Study Participant IDs will be stored in LabKey for cross-referencing purposes.
- Clinic: this field is required. Users will select the proper KCNI site code where the participants collected from a dropdown list. An external link to shows the full name and location of the three-letter site code for reference.
- Sex: this field is recommended as it can be used for quality control purposes in genetic studies. Sex designations specified by the CAMH patient intake form from a dropdown list. For participants where sex was not entered or is irrelevant for the study’s purpose, it is recommended to mark the field as Unknown/Prefer not to answer.
...
- Enter a valid Study Participant ID (numeric values only).
- Indicate the Clinic (collection location) from the dropdown menu.
- Fill the remaining fields as necessary (see table from previous section).
- Ensure that the entered information is correct and in the correct type (refer to the Data Type column in the table above), and press Submit. The window will redirect the user to the Specimen Entry
- Note that pressing the Cancel button will clear the form causing the window to redirect to the Overview tab.
...
In the Participants grid view, each Participant can be edited by selecting the pencil icon to the left of the Study Participant ID column. The user will then be taken to the Update Dataset Entry page with the corresponding Participant ID in the top row.
Each field can be edited by typing into empty text boxes or by replacing existing text. Note that each non-dropdown menu option has a superscript question mark (?) next to the field label. This indicates the expected and required data type that must be entered to update the desired participant information.
Note that the Participant ID and Study Participant ID are uneditable, as changing these values may affect linkages with Specimen and Instrument data. If the Participant is entered mistakenly, or if there are errors while entering the Participant ID, it is recommended to delete the participant record (assuming it is not already connected to any specimen records). If updating the Participant or SP-ID is necessary, please contact the LabKey Adminstrator.
...