Here we outline the process for creating and editing participant records in LabKey for pre-clinical (non-human) studies.
Participants Tab Overview
The Participants tab is organized into two main parts:
- The ‘Enter Participant’ form: this area contains empty fields to enter new participant information
- The Participants grid view: this area shows the entire participant list for the study
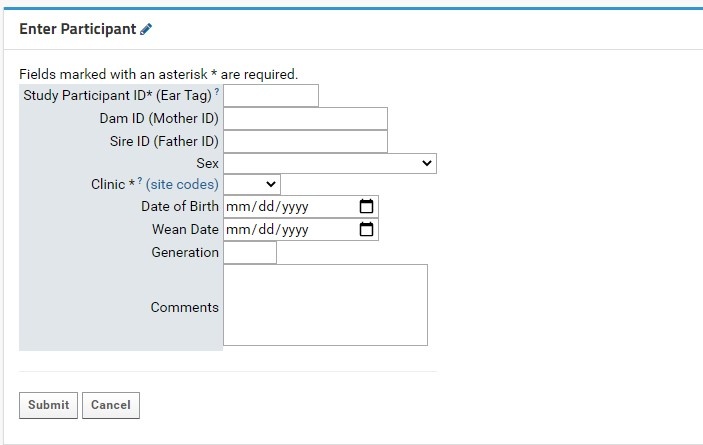
Enter Participants Form
Individual participant entry is done through the Enter Participant form:
- Study Participant ID (Ear tag): this field is required. This field allows for numeric characters only, with a limit of 8 digits in length. This value will be used to generate a LabKey Participant ID, which is standardized across the NIP. A full guide for Participant IDs (LabKey Participant IDs) can be found here.
- Both the Participant and Study Participant IDs will be stored in LabKey for cross-referencing purposes.
- Clinic: this field is required. Users will select the proper KCNI site code where the participants collected from a dropdown list. An external link to shows the full name and location of the three-letter site code for reference.
- Sex: this field is recommended as it can be used for quality control purposes in genetic studies. Sex designations specified by the CAMH patient intake form from a dropdown list. For participants where sex was not entered or is irrelevant for the study’s purpose, it is recommended to mark the field as Unknown/Prefer not to answer.
The following table indicates each possible entry field and their expected data types: