Here we outline the process for creating and editing participant records in LabKey for pre-clinical (non-human) studies.
Participants Tab Overview
The Participants tab is organized into two main parts:
- The ‘Enter Participant’ form: this area contains empty fields to enter new participant information
- The Participants grid view: this area shows the entire participant list for the study
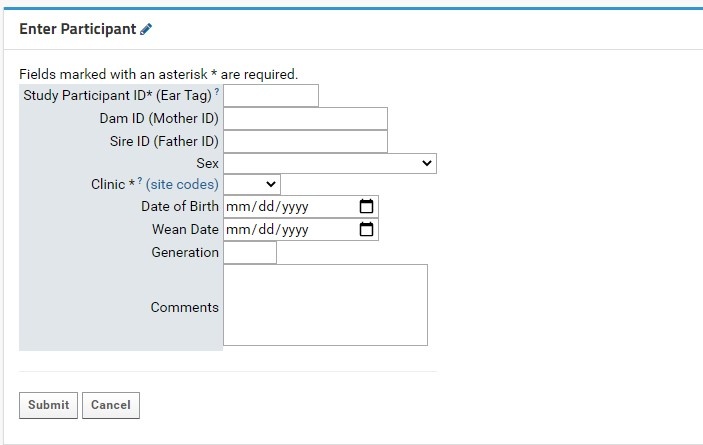
Enter Participants Form
Individual participant entry is done through the Enter Participant form:
- Study Participant ID (Ear tag): this field is required. This field allows for numeric characters only, with a limit of 8 digits in length. This value will be used to generate a LabKey Participant ID, which is standardized across the NIP. A full guide for Participant IDs (LabKey Participant IDs) can be found here.
- Both the Participant and Study Participant IDs will be stored in LabKey for cross-referencing purposes.
- Clinic: this field is required. Users will select the proper KCNI site code where the participants collected from a dropdown list. An external link to shows the full name and location of the three-letter site code for reference.
- Sex: this field is recommended as it can be used for quality control purposes in genetic studies. Sex designations specified by the CAMH patient intake form from a dropdown list. For participants where sex was not entered or is irrelevant for the study’s purpose, it is recommended to mark the field as Unknown/Prefer not to answer.
The following table indicates each possible entry field and their expected data types:
| Field | Data Type | Comments |
|---|---|---|
Study Participant ID (Ear tag) | Numeric (Integer) | Required field. Numeric ear tags will be used to generate NIP Participant IDs that may be used in conjunction with other platform software, should it be required. Each tag must be unique to the project, or else LabKey will not create the new Participant record. |
| Mother ID | Numeric (Integer) | The ear tag of the mother (dam) |
| Father ID | Numeric (Integer) | The ear tag of the father (sire) |
| Sex | Dropdown Menu | Recommended field |
| Clinic | Dropdown Menu | Required field |
| Date of Birth | Date | Can be entered manually, or by clicking the calendar icon and selecting the appropriate date. Expected format: MM/DD/YYYY |
| Wean Date | Date | Can be entered manually, or by clicking the calendar icon and selecting the appropriate date. Expected format: MM/DD/YYYY |
| Generation | Numeric (Integer) | Value must be between 1 and 30 |
| Comments | Multi-line text | Maximum space of 400 characters |
There are two buttons at the bottom of the form:
- Submit will insert the new Participant entry directly into the Participants dataset (assuming all required fields are entered, and optional fields are of the correct data type as indicated in the above table). The user will then be automatically redirected to the Specimen Entry form in the Specimens tab (see Managing Specimens, Specimens in Preclinical Studies).
- The Cancel button will clear the form and return the user to the Overview tab.